June 20, 2006
Food and Drug Administration
Center for Drug Evaluation and Research
Division of Antiviral Products
5901-B Ammendale Road
Beltsville, MD 20705-1266
RE: PIND
number 73,739 - arbidol
Dear Madam or Sir:
I am writing in response to the letter of April 21,
2006 of Debra Birnkrant, M.D., a copy of which is attached at TAB A. The
responses below track the format of Dr. Birnkrant’s letter.
Good Earth Medicine LLC is a company formed for the
purpose of developing research, awareness, and possible marketing of the
antiviral arbidol, which has been used and tested successfully for more than 20
years in Russia for the prevention and treatment of types A and B influenza.
As noted in Dr. Birnkrant’s letter, Good
Earth Medicine is interested in qualifying arbidol for Emergency Use
Authorization for influenza in the event of the declaration of a public health
emergency involving the H5N1 virus or another influenza virus by the Secretary
of public health under Section 319 of the Public Health Service Act (PHS Act).1
1. Summary of the data available regarding the
activity and toxicity of arbidol.
At TAB B to this letter, we have provided copies of
four published scientific papers in
Russian (with professional English translations), as well as two papers in
their original English versions. These papers demonstrate the effectiveness of
arbidol in the prevention and treatment of influenza, including that caused by
the H5N1 virus.
The attached studies also show that arbidol is effective
at a very low level of toxicity. We have noted Dr. Birnkrant’s concern that
an opinion casting doubt about such use of arbidol was received from a testing
laboratory assigned under the NIAID screening program. However, we wish to
emphasize that the testing utilized by the laboratory followed a protocol
different from those shown in the enclosed medical literature, and we otherwise
question why those recent test results were contrary to the results in the
published literature, as attached.2
Arbidol is widely used for prophylaxsis and therapy of
Influenza A and B in Russia. Based upon the long and effective use of arbidol
in Russia, public health officials in that country rely on it as Russia’s primary
defense against the H5N1 virus. According to the Voice of America online
and other publications, President Putin has confirmed that Russia will be using
arbidol as its primary drug for prevention and treatment of avian influenza. A
copy of the Voice of America article is attached at TAB C.
Because of a severe shortage of Tamiflu in the United
States (currently the United States has stockpiled enough Tamiflu for only
about two percent of the population), we believe that the people of United
States would greatly benefit from availability of arbidol to help to counter
the risks of an avian flu pandemic.
Additionally, the recent cluster infections of
H5N1avian influenza in Indonesia has shown that Tamiflu has limited
effectiveness against the strain of virus responsible for those resulting
deaths. It is vitally important to have access in the United States to another
antiviral such as arbidol, which a number of studies have shown to be effective
against influenza viruses including H5N1.
As also requested by Dr. Birnkrant, we have enclosed
at TAB D all reports regarding recent testing results received through the
NIAID screening program.
2. List of additional testing and studies proposed
and plans for development of arbidol.
a. Draft Protocols.
We request that additional testing be done with
respect to the original raw samples of arbidol which we provided for the NIAID
screening program (ARB Numbers 06-000914 and 06-000966), as well as for the raw
sample and the Russian consumer product which we have provided under IND number
73,739. We request that the specific
protocols set forth in the following medical literature attached at TAB B be
utilized:
1. I.A. Leneva, et al., Therapeutic Archives
No. 8 (2005).
2. D.K. Lvov, et al., Emerging Infectious
Diseases (Peer Review, Manuscript ID: EID-05-1609). Please note that in
addition to numerous co-authors at the D.I. Ivanovsky Institute of Virology in
Moscow, the non-Russian co-authors include M. Peiris of the Dept. of
Microbiology of the University of Hong Kong, and D.L. Suarez of the United
States Department of Agriculture, Agriculture Research Service.
3. I.T. Fedyakina, et
al., Vopr Virusol No. 6 (2005).
4. T.A. Semeneko,
et al., Zh Mikrobiol Epidemiol Immunobiol No. 6 (2005).
The protocols used in the above studies resulted in
evidence that arbidol inhibits the reproduction of influenza A viruses, at
concentrations far below the level which is toxic to cells.
b. Plans to Meet Draft Guidelines for Emergency Use
Authorization.
In accordance with the recommendation for data to
support a request for consideration for an EUA (at page 12 of the Draft
Guidance), we submit the following:
1. Description of the product and its intended use.
Arbidol is described as follows, and is intended to be used for the prevention
and treatment of influenza A and B, including forms of the H5N1 virus:3
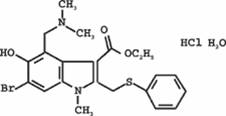
Mesylate of
1-methyl-2-feniltiometil-3-carbethoxy-4-dimetilaminometil-5- hydroxy -
6-bromindola, which has following structural formula:
2. Explanation of unmet need. Avian influenza
type H5N1 is considered by public health officials to present the imminent
potential to mutate into a form capable of causing a pandemic in which millions
of Americans may die. As noted above, the primary line of defense at this time,
Tamiflu, is held in quantities which would leave approximately 98 percent of
the population of the United States without any effective drug coverage to
prevent or treat the disease once contracted. The 2 percent coverage of Tamiflu
which is currently available is not enough to even provide protection to
police, firemen, medical personnel, food transportation workers, and other
essential service providers. Moreover,
in the recent cluster outbreak in Indonesia, Tamiflu is reported to have been
of limited effectiveness. The unmet need in the United States is therefore
enormous, and a drug such as arbidol which has a long history of effectiveness
against influenza A, should be made available to our population.
3. Description of the product’s
approval or clearance status. Arbidol
is not currently approved or cleared under the FD&C Act or licensed under
the PHS Act. An IND has been submitted for raw arbidol under ARB Numbers
06-000914 and 06-000966, with testing results of that product by one testing
laboratory attached at TAB D. Another IND has been established under pre-IND
Number 73,739 for a second batch of raw arbidol and for the Russian consumer
product. As noted in the attached medical literature, arbidol is licensed for
use in Russia, where it has been widely used for more than 20 years with
success.
4. List of manufacturing sites. The
manufacturing sites for arbidol which are known to us are as follows:
a. Jiangyin Eastern Medical
Raw Materials Co., Ltd., No.4 North Road, Huangtu Town, Jiangyin City, Jiangsu,
China 214445.
b. Shchelkovsky Vitamin
Factory, #2 Fabrichnaya Street, City of Shchelkovo, Moscow Oblast, Russia
141100 (for Masterlek).
The manufacture of arbidol is within the regulatory
systems of China and Russia, respectively, and the GMP status of each
manufacturer is not otherwise known to us at this time.
5. Approved alternative products, their
availability, and their adequacy. As noted above, there is a severe
shortage of Tamiflu, which puts virtually the entire population of the United
States at risk. M-2 blockers such as amantadine and rimantadine are other
alternative products, but they have proven to be of limited effectiveness
against certain strains of the H5N1 virus, as have neuraminidase inhibitors
such as Tamiflu in the recent Indonesian clusters.
6. Available safety and effectiveness information
for the product. The following medical research literature attached at TAB
B demonstrates that arbidol is both safe and effective, including against the
H5N1 virus:
i. The literature includes a recent manuscript under
peer review for the journal Emerging Infectious Diseases4, in which the co-authors are not only prominent
Russian scientists, but also scientists from the University of Hong Kong and
from the United States Department of Agriculture. That study found that arbidol
(as well as rimantadine, amantadine, and ribavirin) Aeffectively
inhibited reproduction of HPAI/H5N1 virus in in vitro effective doses
from 1.50 up to 9.70 Fg/ml,
whereas all tested compounds were not toxic up to 40 Fg/ml.@
ii. A 2005 Russian study5
similarly showed that arbidol in the concentration of 10 mcg/ml effectively
inhibits influenza virus A/H5, considerably below the IC50 for arbidol of 40
mcg/ml. Table 1 of that study shows arbidol’s effectiveness as compared to other classes of
antivirals:
Table
1: The influence of antiviral drugs on viral reproduction of various strains of
human influenza
virus
A and B in MDCK cell culture